

 The Accurate Reloading Forums
The Accurate Reloading Forums  THE ACCURATE RELOADING.COM FORUMS
THE ACCURATE RELOADING.COM FORUMS  Guns, Politics, Gunsmithing & Reloading
Guns, Politics, Gunsmithing & Reloading  Gunsmithing
Gunsmithing  Basic Heat Treating of Steel
Basic Heat Treating of SteelGo  | New  | Find  | Notify  | Tools  | Reply  |  |
| One of Us |
Here is the latest installment. I hope that it is understandable, and that the pictures post correctly. Engineers like pictures. At least I do. This covers what changes occur during heat treating, and what it is supposed to do. I will cover Carburizing in another installment. It is a more involved process. Heat Treating: Heat treating has the reputation of being a bit of a black art. There are equations that engineers use to determine times, temperatures, and cooling rates, but at the end of the day, you just have to try it out. To start, what does heat treating do? The short answer is that it makes a soft steel into a very hard steel. Provided the steel is heat treatable. Steel, generally, must have a minimum of 0.25% carbon to be heat treatable. This is an important point to remember. If the steel you have is not heat treatable, or cannot achieve the hardness you want then you have to add carbon to it. This is what is known as carburizing, or case hardening. More on this in the carburizing section. The Detail On Martensite Formation: Steel, like many metals, undergoes changes to its crystal structure upon heating or cooling. Steel has a cubic crystal structure, meaning it is built out of cubes like a brick wall. Each cube is made up of an arrangement of atoms. At room temperature the atoms are arranged in a body centered cubic pattern. See Figure 1.  Figure 1 Body Centered Cubic Atom Arrangement When the steel is heated above 1330F, the body centered cubic arrangement starts to shift to a face centered cubic arrangement of atoms. This is also called the ferrite to austenite transition. Ferrite is the room temperature structure, and austenite is the high temperature structure. See Figure 2.  Figure 2 Face Centered Cubic Atom Arrangement Ferrite, being body centered cubic, cannot hold much carbon (max of 0.02%). The iron atoms are too close to each other for the carbon atoms to fit in. So the carbon exists as iron carbide particles within the steel. When you heat the steel up above the ferrite to austenite transition temperature (nominally 1330F), and it starts to transform to austenite, the iron atoms shift making the gaps between them get larger. Now, the carbon can easily fit between the iron atoms. The iron carbide dissolves and the carbon disperses amongst the iron atoms. If you cool down slowly, the austenite turns back into ferrite, and the carbides reappear. However, if you cool very fast, termed quenching, a whole new structure is formed. Martensite. The mechanism for martensite formation works like this. The steel is at high temperature (>1330F), and the carbon is dissolved and dispersed within it. It takes time for the carbon to congregate and form a carbide when you cool the steel down. If you don’t give it enough time to congregate, it becomes trapped within the structure and distorts it. This distortion makes the steel extremely strong and hard. This new structure, called martensite, is what we are after in heat treating. Martensite is very hard and strong, but when it is newly formed and 100% of the carbon is trapped, it is also very brittle. Drop the part or hit it with a hammer, and it will shatter. To make it tougher, we heat it up to lower temperatures, 400F to 1300F, for a short time and allow some of the carbon to migrate out of the martensite and create small carbide particles, called temper carbides. This relaxes the structure a little, lowers the hardness, lowers the strength, and increases the toughness. This is referred to as Tempering or Drawing. This is critical to achieving both a strong and tough part. The maximum hardness and strength of the martensite that you can obtain is directly related to the amount of carbon present in the steel. See Figure3. 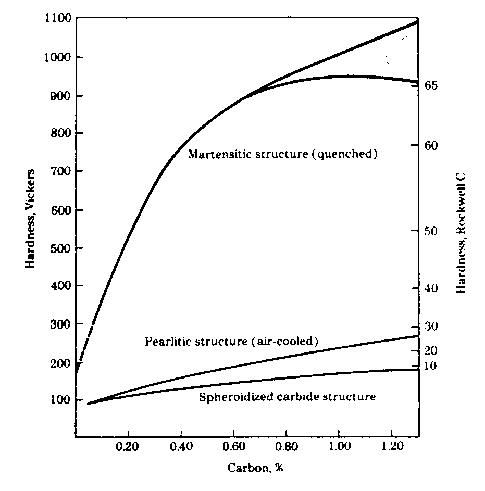 Figure 3 Hardness of as Quenched Martensite as a Function of Carbon Content Martensite can be formed intentionally through a heating, and quenching process. Or it can be formed as a by product of another process. Commonly, welding and grinding operations inadvertently produce martensite. Martensite formed in this way is typically a bad thing. It is untempered, and very brittle, making the weld or ground area brittle as well. In many cases, the heat affected zone near the weld bead will crack because of the martensite formed there. As the weld cools, it contracts and puts the weld in tension. The martensite is too brittle to withstand the tension stress, and it cracks. The cracks will run parallel to the weld bead on either side. This is common on high carbon and alloy steels. Special welding practices need to be followed in order to weld these materials. Usually, this involves pre-heating of the part, and using a heating torch to slowly cool the weld area. Remember slow cooling prevents martensite formation. So to summarize this section. Steel is at its heart built of cubic crystals. Upon heating or cooling past 1330F, the cubic crystals undergo an atomic arrangement change and either can hold carbon or cannot. If the steel is held above 1330F and all of the carbon is evenly dispersed throughout the steel, then it is plunged into a quench media (water, brine, oil) and cooled rapidly, martensite is formed. Martensite is hard, strong, but brittle, and must be tempered to make it tougher. Thoughts on Heat Treat Design: The heat treat cycle must be designed for the most critical area of the part when in service. This means that the heat treat cycle is designed to properly harden the receiver lugs not the tang. It doesn't do you much good if the lugs aren't hard enough or are too hard. The parts must be heated uniformly, and they must quench uniformly. Pay special attention to how the parts are placed into the furnace, or crucible. Don't let them touch, don't let them shield each other from the heat. When the parts are dumped into the quench they need to be agitated so that they don't lay on each other and prevent proper cooling in any area. Stirring them or shaking them in a basket is a simple and effective approach. The heat treat cycle must minimize distortion of the part. This mostly has to do with the quench media that you use. Plain water is a fast quench, but it isn't a uniform quench. Brine is faster than water, and can be more or less uniform. Oil is slower, and very uniform. Other quenchants like polymers, salts, gas jets, etc. have been developed to reduce distortion in specialized situations. Quench media should be chosen to achieve the cooling rate without distorting the part or quenching too fast. If the quench is too rapid, the part will develop cracks. For most steels, oil (motor oil works fine) is an effective choice. Water is good for small parts, and simple parts. Water forms a steam blanket around the part until the part cools below the boiling point. This steam blanket can prevent proper quenching in corners, and pockets. Oil doesn't have this issue. Brine (water plus almost any sort of salt: NaCl, KCl) quenching is faster than water. It is good for small parts that are in the 0.25% to 0.35% carbon range where a fast quench is required. Otherwise, it isn't suitable. The part must be rinsed in clean water right after this so that it doesn't rust and pit. Keep in mind that the quench media volume should be fairly large. You don't want the part to heat the quench media up a lot. It isn't an effective quench if the part is still smoking hot when it is removed from the quench. For a part the size of a receiver, 4-5 gallons is a good rule of thumb. Heat Treating High Carbon and Alloy Steel: This is a relatively simple process. These steels are designed for this, and generally it is straightforward. Step One: Choose a temperature to heat the steel to that will allow it to become 100% austenite. You do this using the iron/carbon phase diagram, but that exercise is beyond my wordsmithing skills. Thankfully, being exact isn't critical here, generally heat treaters use a temperature of 1550F for steels with 0.35% to 0.50% carbon, and 1450F for steels from 0.50% to 0.80% carbon. The steel must be held at this temperature for a minimum time to allow the austenit to form, and to allow the carbides to fully dissolve. Times range from seconds to 1 hour or so depending on carbon content, and part size. On parts with ¼ inch thick sections, start with 30 minutes and see if you get to the max hardness for the carbon content that you are working with. On thin springs and the like a few seconds is sufficient. Step Two: Remove parts from furnace and immediately dump them into the quench media. Let them cool to room temperature in the media. Step Three: Set the furnace to the tempering temperature, place the part or parts in, and hold for one hour. Remove the parts and let them air cool. The tempering temperature is chosen based on the carbon content and the desired final hardness. See Figure 4. All heat treated parts should be tempered at 450F for an hour at a minimum. This is termed a safety draw, and it prevents the part from catastrophically failing, i.e. exploding. Heat treating builds a lot of stress in the part, and they can come apart for no good reason. 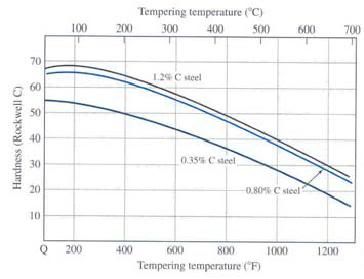 Figure 4: Hardness of martensite tempered one hour as different temperatures Step Four: Check the hardness on the Rockwell C scale, and verify that you got what you expected. If not, temper again, or re-heat treat with appropriate changes. That pretty much covers it. Thank you for your time, and if you got this far, treat yourself to a beer, you deserve it. Jeremy | ||
|
| One of Us |
Holy smokes!!! The pictures showed up. | |||
|
| One of Us |
Great job of explaining the process, very easy to follow. Can't wait for the case hardening installment. Thanks for doing this. Steve | |||
|
| one of us |
You're explaining it well ! | |||
|
| One of Us |
What about Cryo-treatment? | |||
|
| One of Us |
You are doing this in a great manner, farbedo! Very easy to understand even for the totally uniniciated peopple. Thank you very much and will waint for the followings !!! Regards PH | |||
|
| one of us |
hawkins , I can give you the latest on that and sift out the BS [lots of that]. Benelli has has photos of structure of cryo'd and uncryo'd barrels .Except that it's nonsense ! To be technical here there are two cold treatments . Subzero which has been around for many years is - 100 F which is what Co2 [dry ice ] will give you.This will reduce the amount of retained austenite. Cryogenic cooling is the newer process and it the temperature of liquid nitrogen ,- 300 F. This will remove even more RA and alter the crystal structure a bit to permit the formation of fine carbides called eta carbides on susequent tempering. Retained austenite [RA] is a problem with high carbon ,high alloy steels not low carbon low ally steels. Other claims are highly suspicious !!! As far as rifle or shotgun barrels and accuracy, life etc the NRA found no difference and a number of excellent makers also found no difference ! This is the latest info.I have had these discussions many times on knife making forums so I've been carefully studying it. | |||
|
| One of Us |
My own personal experience with CRYO makes a barrel machine better and easier. I have not seen any other advantage to it. I believe Kreiger uses it for savings in tooling cost and I don't believe they claim otherwise. Butch | |||
|
| One of Us |
Farbedo, what is your experience with M70pre64 and military mausers actions, have you experienced brittleness,lug setback or any other problems? in your view is re-treat advisable for modern High pressure chamberings? | |||
|
| One of Us |
Another great post. I knew perhaps 85% of this, but never completely understood the "carbide solution" stuff as related to "slack quench structures" and the like. Now it's much clearer. Thank you. | |||
|
| One of Us |
went over it years ago in a "strength of materials" class (Lakeland Com Col)- surprised at how quickly it got familiar, and how much more I'd like to review it again. good info in both threads. used to be (still?) a place in Cleveland (grew up there) called the Eutectic and Castolin Institute on the south side of the shoreway in Collinwood, and if you called them with a welding/metal problem, they'd bend over backward to help solve it, usually coming to where you and the problem were with material and information. | |||
|
| one of us |
I cannot find any mention of cryo treating barrels on the Krieger website. Has the fade finally gone away ?? | |||
|
| One of Us |
Unless they just recently changed,, they are still CRYO. Butch | |||
|
| One of Us |
Very interesting information. I'm no gunsmith - not by a long shot - but I have made some parts, such as flat springs, and tools, such as screwdrivers and scrapers, that required hardening by heating, quenching and tempering. Before reading this, I never really understood the science behind what I was doing. When I did my heat treating work, I did some reading, which was more practical than scientific. The authorities I consulted said to start with steel that had good carbon content, heat it cherry red, quench it in oil, then re-heat it to a straw color and allow to air cool. It took me about four tries on my first job. The work was coming out either too soft or too hard and brittle. But I got it done, finally. It seemed to be as much art as science! Thanks for this, Jeremy. Mike Wilderness is my cathedral, and hunting is my prayer. | |||
|
| One of Us |
I think the basic thing people want to know, on top of what has already been provided, is exactly what steel was used to make their action. | |||
|
| One of Us |
Being in Aerospace Heat Treating is one of our critical processes and you could write books about what people do not know about the process and what it does. The key here is that the Heat Treat Facility knows exactly what it is doing and that the process is strictly adhered to. Every part that comes into the facility that has required a heat treat in the manufacturing process is certified to and traceable back to the individual process operation or lot. Our approved processers are controlled and when a mistake is found and it does happen there are potentially dire consequences including the grounding of aircraft. | |||
|
| One of Us |
They have/had a place on long island. Very helpful folks. My buddy used to print their catalogs. [QUOTE] Eutectic QUOTE] | |||
|
| one of us |
this is answering about a million questions for me...most of which i was too ignorant to even ask. i submit that farbedo needs his own forum. i mean, if we have a forum for gun cleaning, certainly a metallurgy forum is worthy and would come in handy:-) i'm only half kidding :-) blaming guns for crime is like blaming silverware for rosie o'donnell being fat | |||
|
| One of Us |
Krieger offers two cryo's. One is done early in the barrelmaking process and the other is done after completion and is an option. Jim Kobe 10841 Oxborough Ave So Bloomington MN 55437 952.884.6031 Former Professional member American Custom Gunmakers Guild | |||
|
| One of Us |
You are right Jim. I remember know. Butch | |||
|
| Powered by Social Strata |
| Please Wait. Your request is being processed... |
|

Visit our on-line store for AR Memorabilia

