

 The Accurate Reloading Forums
The Accurate Reloading Forums  THE ACCURATE RELOADING.COM FORUMS
THE ACCURATE RELOADING.COM FORUMS  Rifles
Rifles  Medium Bore Rifles
Medium Bore Rifles  Shelf life of centerfire ammo?
Shelf life of centerfire ammo?Go  | New  | Find  | Notify  | Tools  | Reply  |  |
| One of Us |
I'm thinking about buying in bulk for the great prices then store the ammo and use it over years. Does ammo deteriorate over time? Talking about powder charge taking moisture, becoming less effective, or even the brass itself oxidizing. What can you tell me about that, and how long can I expect stored ammo to posses 100% factory performance? Thank you Don't Ever Book a Hunt with Jeff Blair or Blair Worldwide Hunting http://forums.accuratereloadin...043/m/3471078051/p/1 | ||
|
one of us |
I am still shooting 1950s vintage factory rifle & shotgun ammo w/o noticeable deterioration. NRA Life Member, Band of Bubbas Charter Member, PGCA, DRSS. Shoot & hunt with vintage classics. | |||
|
| one of us |
Every few years I'll fire a round of .45 ACP from a box that my father brought back from WW-II over the chronograph. It clocks just the same as it did forty years ago when I first started doing this. It has had no special storage other than being inside the house. So, do you anticipate using the ammunition you buy today more than 70 years from now? If so, you might want to reconsider your hoarding plans since I have no data on ammunition older than about 70 years. | |||
|
| One of Us |
Old age is a high price to pay for maturity!!! Some never pay and some pay and never reap the reward. Wisdom comes with age! Sometimes age comes alone.. | |||
|
| One of Us |
I'm 46 soon enough I'm not at all worried about ammo, powder or primer shelf life ________________________________________________ Maker of The Frankenstud Sling Keeper Proudly made in the USA Acepting all forms of payment | |||
|
| one of us |
I would say that it all depends on the components used by the manufacturer. I too am a hoarder and have factory ammo purchased and stashed, some for over 40 years. It also has to do with the ambient temperature. Occasionally, I find "old ammo" from the early 90's where the powder has deteriorated and the acid residue has corroded the brass case from the inside. Sometimes the powder breaks down and only weeps internally but kills the primer. So, frequent misfires are common. Again, previously accurate ammo lots start to shoot erratically in the same rifle. This may imply that the powder is deteriorating affecting the burn rate. Then again, I have some very old Rem. green box 30-06 180 gr. match ammo from the 60's that is currently the most accurate factory stuff I have. All of my factory ammo has been stored in G.I. ammo cans since acquisition. Surely, my experiences are shared by other old timers here. Geoff Shooter | |||
|
| one of us |
My brother still hunts with 25.06 reloads that were made in the 1960s. Frank "I don't know what there is about buffalo that frightens me so.....He looks like he hates you personally. He looks like you owe him money." - Robert Ruark, Horn of the Hunter, 1953 NRA Life, SAF Life, CRPA Life, DRSS lite | |||
|
| One of Us |
Old age is a high price to pay for maturity!!! Some never pay and some pay and never reap the reward. Wisdom comes with age! Sometimes age comes alone.. | |||
|
| One of Us |
Most of the answers you have read in this thread are based on confirmation bias. Shooters don’t want to believe that their ammunition won’t last forever, so they ignore all evidence that it won’t. Of course ammunition has a shelf life, and it is primarily based on the lifetime of gunpowder. Federal says their ammunition has a ten year shelf life: Federal Ammunition : http://www.federalpremium.com/company/faq.aspx
Early in the last century the storage lifetime of smokeless powders was considered to be 20 years or less: Army Ordnance Magazine, June 1931, page 445 says:
Nitrocellulose is a high energy molecule that is breaking down to become a low energy molecule. Anyone who has taken thermodynamics will realize that this is obvious, everything is breaking down to a lower energy state, but somehow, shooters have been lulled into thinking that the second law of thermodynamics does not apply to our sport. However, no shooter/reloader has given a good reason why out of all of the elements, compounds in the universe, why only ammunition is immortal. I suspect this is because they don’t want to think about it and therefore don’t. I can’t say it any better than the Dec 2003 Propellant Management Guide: Stabilizers are chemical ingredients added to propellant at time of manufacture to decrease the rate of propellant degradation and reduce the probability of auto ignition during its expected useful life. As nitrocellulose-based propellants decompose, they release nitrogen oxides. If the nitrogen oxides are left free to react in the propellant, they can react with the nitrate ester, causing further decomposition and additional release of nitrogen oxides. The reaction between the nitrate ester and the nitrogen oxides is exothermic (i.e., the reaction produces heat). Heat increases the rate of propellant decomposition. More importantly, the exothermic nature of the reaction creates a problem if sufficient heat is generated to initiate combustion. Chemical additives, referred to as stabilizers, are added to propellant formulations to react with free nitrogen oxides to prevent their attack on the nitrate esters in the propellant. The stabilizers are scavengers that act rather like sponges, and once they become “saturated” they are no longer able to remove nitrogen oxides from the propellant. Self-heating of the propellant can occur unabated at the “saturation” point without the ameliorating effect of the stabilizer. Once begun, the self-heating may become sufficient to cause auto ignition. Actually there are only a few compounds used as stabilizers, and as the Propellant Management Guide tells us, stabilizers are consumed with age. This is a good reference on stabilizers, ROLE OF DIPHENYLAMINE AS A STABILIZER IN PROPELLANTS;ANALYTICAL CHEMISTRY OF IPHENYLAMINE IN PROPELLANTS
http://www.dtic.mil/dtic/tr/fulltext/u2/783499.pdf Heat is used to accelerate aging of the powder and as the powder ages, the stabilizer content decreases. 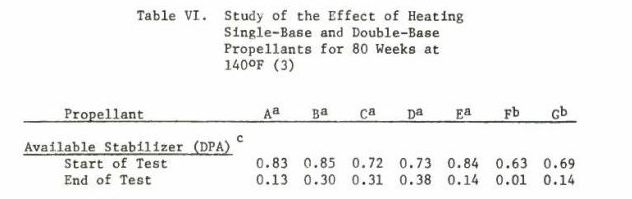 The bitter smell and red color (probably nitric acid gas) we see and smell in very old gunpowder is a consequence of not enough stabilizer left to sop up all of the NOx. As NOx escapes it reacts with water to produce nitric acid gas. That nitric acid gas corrodes brass, bullets, weakens brass, is evidenced by cracked case necks, eats up the ammunition containers; nitric acid gas is nasty stuff. I am certain that a cloud of red fuming nitric acid gas is as toxic as any of the chemicals used in WW1 for chemical warfare. Our Armed Services have stockpile surveillance programs (but each Service does theirs a little differently) and one of the easiest things to show that gunpowder is at the end of its service life is that red fuming nitric acid gas. Of course there are a lot of tests, if you want to see all the different tests the military uses look at Mils Std 286 Propellants, Solid: Sampling, Examination and Testing to be found at https://assist.daps.dla.mil/quicksearch/. One common test for gunpowder age is placing the suspect powder in an oven at 65 C (150 F) until it fumes. If the sample fumes within 30 days the lot in the field is either chemically tested for the percentage of stabilizer or it is simply scrapped. This is from a 1969 Symposium: 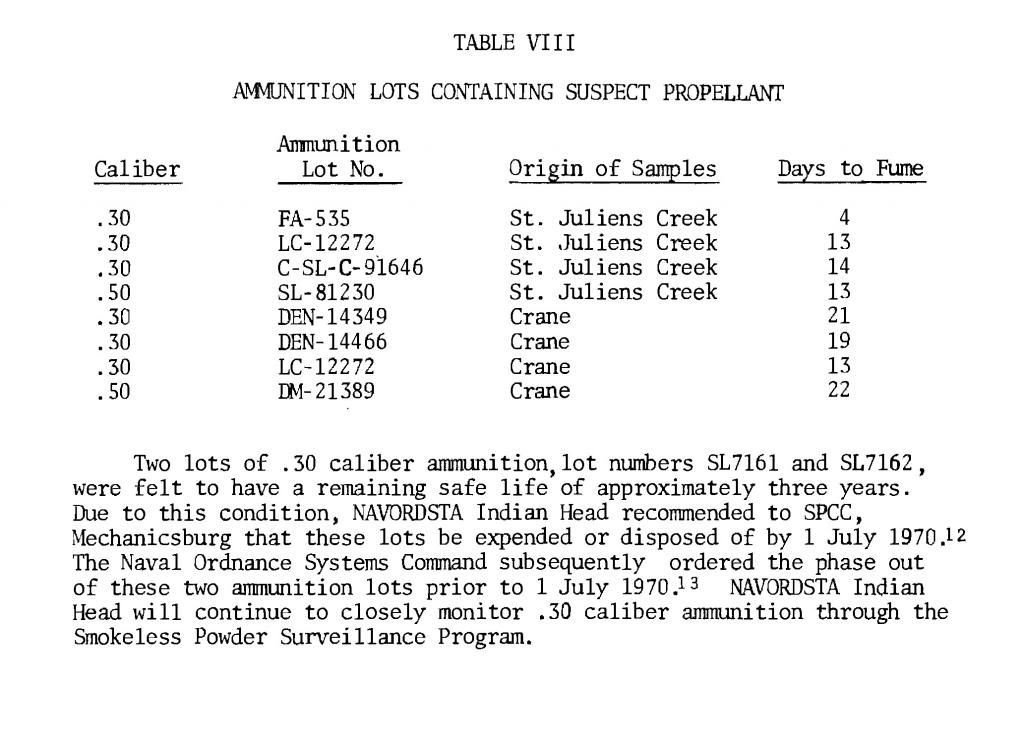 This is from a 1970 Symposium: 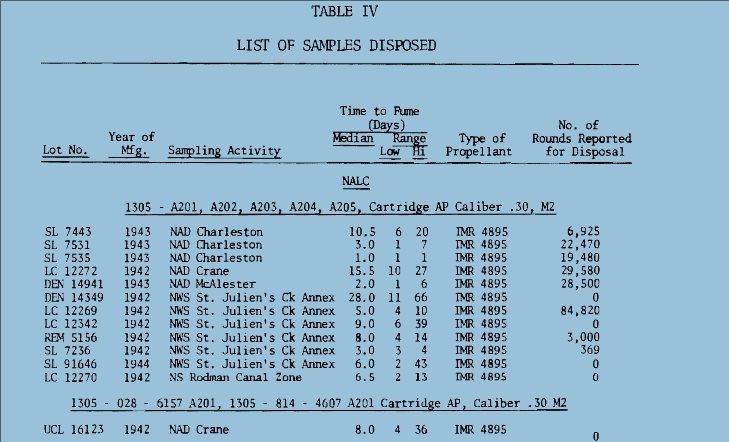 Mind you the WW2 ammunition they were scrapping in 1969 was around 26 years old. So, when people claim they have 70 year old ammunition, what does that tell you about the probable condition of their ammunition? Each service has its own peculiarities, the Navy expert told me they keep master samples in test tubes. In the test tube is a methyl violet paper, if the paper changes color, they track down the powder lot and test to see how much stabilizer is left. If the amount is less than or equal to 20%, the lot is scrapped. I think this is called the Methly Violet test, or Talliani test in Mil Std 286. The Army must do something similar because page 5-11 of the 2003 Army Logistics Propellant Management Guide provides the protocols for their Stockpile Propellant Program. The trigger for investigation is: "When Master Sample Stability Failure Occurs" The Navy and Army are consistent in that they scrap powders and propellants when the stabilizer is decreased from 100% to 20%. So, what do you do if you don’t have a chemistry lab to check the percent of stabilizer? Well all you have left is the gross indications of seeing fuming nitric acid and smelling a horrible bitter smell. The smell will knock your socks off. If you see or smell fuming nitric acid the powder went bad long ago. The stuff is absolutely unsafe to shoot and unsafe to store. More on the second point later. A Navy expert provided 'rules of thumb' concerning the safe lifetime of double based and single based propellants. The rules of thumb are: Double based powders and ammunition are scrapped at 20 years, single based 45 years. In his words “These 'rules of thumb' are particularly useful when the protocol fails. The protocol can easily fail when workmanship or good housekeeping measures are not followed during manufacture of propellant and/or rocket motor or during storage of the weapon system components, respectively.” I want to say, given hot storage conditions, sloppy manufacture, the lifetime of your gunpowder can vary considerably. Rules of thumb are best guesses and best guesses are guesses. Sometimes best guesses work out the way you predicted, when they do people will call you a prophet and you will pat yourself on the back for being a genius. When best guesses go wrong, you will wear the dunce cap and wonder what that word “hubris” means. Heat accelerates the deterioration/decomposition of powder and the rate is directly proportional to the Arrhenius equation, which is an exponential function. Maybe this is a simpler way to say it: the lifetime of ammunition decreases exponentially as temperature rises. This table is instructive on how quickly heat deteriorates smokeless propellants: UN manual on ammunition inspection. See section 7.3. [b]Surveillance and in-service proof - the United Nations[/B http://www.un.org/disarmament/...ice%20Proof(V.1).pdf 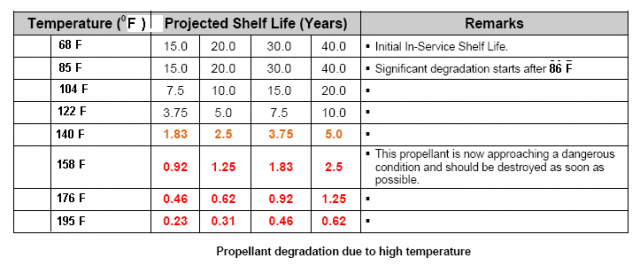 If you read Insensitive munitions literature, you will see that they use high temperature to accelerate aging of smokeless propellants. As gunpowder gets older it gets more dangerous to shoot. Old gunpowder will, and has, blown up firearms. The basic reason is something called “burn rate instability”. You want a nice and smooth pressure curve. If the burn rate is irregular, because the nitrocellulose powder grain breaks down irregularly, there will be peaks and valleys instead of a smooth pressure curve. These irregularities can interact in such a way that pressures spike. Double based powders are a combination of nitroglycerine (NG) and nitrocellulose, the NG is there for an energy boost, but unfortunately NG causes a new set of problems. NG is apparently not bound to the powder grain but is a liquid and it migrates. NG is wicked to the surface of the powder grain over time, one causal reason, water condensing and evaporating on the powder grain surface. The evaporating water molecules pull on the NG. I was told that created a NG rich surface. So, even though the total energy of the grain has decreased due to breakdown, the surface is NG rich and that will spike the initial burn rate. Another thing NG does is accelerate the breakdown of the base nitrocellulose molecule by attacking the double bonds holding the NO molecules. Unfortunately all ionic compounds attack those double bonds, water is a main offender because it is always in air, is a polar covalent ion (acts like an ionic compound) and thus you know the reason you were told to store gunpowder in a cold and dry environment. Quality ammunition is manufactured in humidity controlled environments, between 40% and 20% humidity, but they don't go lower due to electro static discharge concerns. Incidentally rust is bad and that rust that came out of those old tin cans accelerated the aging of gunpowder, and I think, is why they went to plastic containers. There is almost no data on the internet because all that was ever needed to be known about gunpowder aging was determined well before WW2. However ball powders did come out at the end of WW2 and I was able to find this data showing that gunpowder at the end of its lifetime will pressure spike. I asked you remember that heat is used to accelerate the age of gunpowder, so what you are seeing is in fact because of “age”, not heat, but it took heat to age the powder quickly. The IMR is a single based and the WC is a double based ball powder. INVESTIGATION OF THE BALLISTIC AND CHEMICAL STABILITY OF 7.62MM AMMUNITION LOADED WITH BALL AND IMR PROPELLANT Frankfort Arsenal 1962 3. Effects of Accelerated Storage Propellant and Primer Performance To determine the effect of accelerated isothermal storage upon propellant and primer performance, sixty cartridges from each of lots E (WC 846) and G (R 1475) were removed from 150F storage after 26 and 42 weeks, respectively. The bullets were then removed from half the cartridges of each lot and from an equal number of each lot previously stored at 70F. The propellants were then interchanged, the bullets re-inserted, and the cases recrimped. Thus, four variations of stored components were obtained with each lot. Chamber pressures yielded by ammunition incorporating these four variations were as follows. These values represent averages of 20 firings. 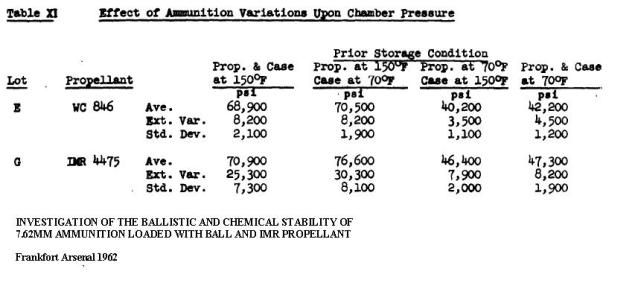 So to summarize, powder does not get better with age, heat ages powder fast, old gunpowder will blow up your firearm. Anyone with a $100,000 dollar irreplaceable machine gun should seriously consider the practice of shooting cheap surplus military ammunition. Machine gunners shoot orders of magnitude more ammunition than most people, so as they are burning through a pallet of surplus ammunition, they are more likely to find that statistically improbable bad round. A machine gunner bud of mine told me he had “blown the top cover twice” with 50’s Yugo 8mm. After I told him about pressures and old ammunition, he said it all made sense. Of course if you are one of those who compulsively hoard ammunition, information on the thermodynamics, kinetics, chemistry of gunpowder won’t change your mind or your behaviors. | |||
|
| One of Us |
Bullet weld and caked and/or oxidized powder are two of the biggest problems with older loaded ammo. Both situations are no bueno. Best to carefully store and carefully inspect each round of older ammo before use. Not a bad idea to pull a bullet or two to check for bullet weld and/or deteriorated powder. Test firing is a little sketchy... ___________________ Just Remember, We ALL Told You So. | |||
|
| One of Us |
I received permission from the poster to use his pictures and text to warn others. If your ammunition ever looks like this, I would recommend not shooting it. Corrosion like I have never seen http://thefiringline.com/forum...wthread.php?t=542632
    | |||
|
| One of Us |
Slam Fire; That is very interesting. My storage concern is not so much about heat but rather extreme cold. I wonder what the affect of-40° each winter would be on the stored powder and stored ammo?? Phil Shoemaker : "I went to a .30-06 on a fine old Mauser action. That worked successfully for a few years until a wounded, vindictive brown bear taught me that precise bullet placement is not always possible in thick alders, at spitting distances and when time is measured in split seconds. Lucky to come out of that lesson alive, I decided to look for a more suitable rifle." | |||
|
| One of Us |
The Navy insensitive munitions expert told me the ideal storage conditions were Arctic: Dry and unchanging cold. It is worth examining this document for a reality check on storage in cold conditions: An Examination of Deterioration of Ammunition by Storage. NORWEGIAN DEFENCE RESEARCH ESTABLISHMENT http://oai.dtic.mil/oai/oai?ve...identifier=ADA055897 Norway is cold, by CONUS standards, and yet you will see munitions deterioration even in this climate. If you want to keep ammunition for a long time, start off with new ammunition, not surplus ammunition or powder that has been discarded because it was at the end of a reasonable shelf life. Then, keep it cold and dry. I am skeptical whether absorbent materials, such as desiccants, will extend the lifetime of ammunition. If the gunpowder is in the case, whatever water is there will stay there. Heat is by far the worst enemy of gunpowder. Primers will last longer than gunpowder, but high temperature will dud them out, at least I have found references to "high temperature" primers. The storage lifetime of primers is much longer than gunpowder, and unlike gunpowder, they dud out. Gunpowder will auto combust and is therefore more hazardous to store in bulk. Gunpowder in small arms, it is a bit of a argument whether it will auto combust. One group claims that the brass case acts as a heat sink and will prevent auto combustion in ammunition smaller than five inch artillery. Others claim this is bogus. All agree that old gunpowder in bulk, such as decanting old gunpowder and storing it in large containers, like 8 lb kegs and up, is an auto combustion event waiting to happen. Hoarders proudly post pictures of the WW2 era Hodgdon powder containers they have in their houses. These people have no idea that they may wake up with their house in flames!!   As stated earlier, exposure to ionic or polar covalent materials is bad for gunpowder, and will, of course, eat through the sidewalls of a brass case. | |||
|
| one of us |
Oh gawd - I just had a flashback to Thermo class! | |||
|
| one of us |
I make no such claim; it is a fact that the ammunition I referenced is at least 70 years old. It is also a fact that this ammunition consistently (from year to year, approximately a half-decade apart) chronongraphs around 850 FPS with its (presumably) 230 FMJ over an Oehler Model 35. This is the most extreme example I can cite of old ammunition behaving exactly as it did when it was first manufactured. However, I have other examples, including "fresh" ammunition loaded only last week, but with 70 year-old Surplus 4831 and 40 year-old RWS Sinoxid primers. The cases are youngsters, being Herter's from the 1970's; but the bullets are virtually new, having left the factory during the first couple of years of the current century. And then there is the stack of Herter's .222 factory loads . . . I realize that all of these are anecdotal examples, just as the ammunition or components cited in previous posts are anecdotal examples of deterioration. Of course deterioration happens -- every molecule has a half-life. And no batch of components is identical to any other batch, nor are they loaded the same way and under the same conditions. My point is that the deterioration of modern ammunition is a very slow process which usually spans more than the lifetime of an individual human. Certainly not every piece of ammunition nor its component parts may remain serviceable as long as this. Ammunition and components manufactured during the "rush" of the most recent shortage scare might indeed be suspect due to possible manufacturing shortcuts. But typically, ammunition has a useful lifetime greater than that of its owners. | |||
|
| One of Us |
Can relate to pics posted by Slamfire. Had some surplus ammo for 7.65x53 made back in the 40s, that I'd bought back in the late 60s. Had it stored in a closet in my house and long since forgotten about. Came across it about 7 years ago when looking for something.. Much of it I could easily twist off the bullet at neck/shoulder.....had corroded thru at neck/shoulder. Also had some CCI minimag 22 ammo from the late 60s in the box stored in the closet. I would shoot around 10 or so rounds of it at range. About every third round fired, brass case had a long split in side of brass. I threw the rest away. | |||
|
| One of Us |
Man... I been shooting up a truckload over the years of 1939 German 154gr FMJ ball made for Turkey.. Stuff looks & shoots like it was made yesterday.. Down to my last 800rnds or so.. Same with WWII 30-06 I also had some 7.65 made in the 60's that just fell apart, I ended up just pulling the bullets.. MopaneMike | |||
|
| One of Us |
In 1979 I bought an absolutely mint Trapdoor from a shop in Boise. Moon's for those of you old enough. Malt shop and guns in the back. I was discussing the total lack of ammunition for the old 45-70. Bernie (Moon) takes me down a flight of stairs to the basement. His stash dated back to the late-nineteenth century. We pull boxes out of the way for an hour, and finally uncovered a crate of Frankford Arsenal black powder rounds with an issue date of 1884. iirc, 840 rounds. I buy the case for $100, and proceed to take it all out and shoot it over a six month period, including big game hunting. Every round fired. Soooooooo, I would not worry about any stuff not corroded green. Rich | |||
|
| One of Us |
Very interesting. Speaks well of the primers. And the powder? Was it blackpowder? Blackpowder is an entirely different propellant than smokeless and any issues associated with smokeless are entirely different. Blackpowder is very stable and periodically I read of Civil War cannon balls killing a collector.
You have ignored anything that does not support a very long lifetime for gunpowder. You completed passed over the comments by Picatinny Arsenal and the scrapping of 26 year old WW2 ammunition. You have no physical, chemical basis for your statement that gunpowder usually spans more than the lifetime of an individual human. And how long is that? Human lifetime is very unpredictable in itself . Lots of children, teenagers, young people in their 20's. 30's, die unfortunate early deaths. I worked in one office for 15 years and it was surprising out of 200 co workers, how many died of cancer, one a 40 year old mother, others in their 50's. There were a lot of causes, but cancer killed the most. These were not octogenarians, hardly any of them were in their 60's as most retired before 60. People focus on averages (77 years or so) and ignore the number of people they knew who died well before 77. It is unfortunate that gunpowder does not have a predictable shelf life. First world countries pay massive amounts of money in stockpile surveillance. They want to get that ammunition out before it auto ignites. Second and third world countries have the habit of letting the Munitions Dump blow. The US paid $100's of millions of dollars removing old ammunition from eastern European countries because ammunition dumps were starting to explode. People don't have the slightest idea the quantity of munitions that is scrapped every year, scrapped because it is too dangerous to store and too dangerous to issue. Weapons Collection and Destruction http://www.smallarmssurvey.org...icant-surpluses.html Industrial Demilitarization of Conventional Ammunition http://www.smallarmssurvey.org.../highlight-rn37.html In 2005 Congress wrote this in one of their funding documents.: the Department of Defense plans to increase spending on conventional demilitarization to approximated $541 million for fiscal years 2006 through 2011 to reduce its current backlog of approximately 390,000 short tons. https://books.google.com/books...%20munitions&f=false Their backlog of undestroyed munitions was 390,000 short tons! The Army destroys around 1.2 Billion dollars of munitions a year! http://www.usatoday.com/story/...r-gao-waste/8145729/ The Army was criticized because they have so much old stuff, and users are unable to identify what is in stockpile and use it before it goes bad. Here is a nice recent example of 20 year old factory ammunition going bad. Remington 700 Overpressure with 20 year old factory ammunition http://thefiringline.com/forum...wthread.php?t=527519
One should ask, how could any military justify making ammunition with a 100 year shelf life. I don't know if it is possible to require ammunition to have a 100 year shelf life. I expect there would be undesirable trade off's. Even if the ammunition lasted 100 years, it is the rare service weapon staying technologically advanced enough to issue 100 years after manufacturer. The Brown Bess is an oddball example that was issued for 300 years, but it was not a cartridge weapon. The history of service rifles post American Civil War is a history of service rifles being totally obsolete 20 years after adoption. How long did the 1861 musket stay in service? It was replaced in 1873. Martini Henry rifles, Remington rolling blocks, and M1871's were issued to Colonial Troops during the first World War, but they sure were not front line weapons on the Western Front. The family history of the M71 was that Great Grandfather carried it during WW1 while serving in the German Landwehr. As I understand, these guys guarded train bridges, canals, etc. Look at all the proof marks.    Prior to Great Grandfather lugging this M71, the German Army was scrapping ammunition at 10 years clock time. They had established that pressures rose after ten years and they were selling ten year old ammunition to unsuspecting buyers, telling them that the ammunition had been tested and was perfectly safe at the time of the test. What they did not tell the buyers of this surplus ammunition, was that the ammunition had been tested and the test results indicated the ammunition had reached the point where it becoming dangerous to shoot. Shooters of old ammunition should realize that they are shooting ammunition that the maker never had an expectation of 50, 70, 100 year shelf life. First World country militaries pay people to go through their ammunition stocks and weed out the old, dangerous, munitions. You come around and trade your Cow for a handful of magic beans, but this is not a fairy tale, and no magic golden harp will be found. Instead, with old ammunition, you are playing an advanced game of Russian Roulette. You don't know what the pressures are in the case, you don't know how deteriorated the gunpowder in that case, and you don't know, if the rounds are good or bad, until your gun blows up in front of your face. Many shooters find this process totally acceptable, and totally ignore the risks, to help them justify their actions. | |||
|
| One of Us |
Why shoot old ammo when the new stuff is so much better? | |||
|
| one of us |
Properly stored it seems to last forever..I ran out of primers a while back and found some 1950 CCI primers and 200 roundes 222 ammo with them..Shot all that stuff up at our rock chuck population to get rid of it, it was accurate and I had no misfires.. Ray Atkinson Atkinson Hunting Adventures 10 Ward Lane, Filer, Idaho, 83328 208-731-4120 rayatkinsonhunting@gmail.com | |||
|
| Powered by Social Strata |
| Please Wait. Your request is being processed... |
|
 The Accurate Reloading Forums
The Accurate Reloading Forums  THE ACCURATE RELOADING.COM FORUMS
THE ACCURATE RELOADING.COM FORUMS  Rifles
Rifles  Medium Bore Rifles
Medium Bore Rifles  Shelf life of centerfire ammo?
Shelf life of centerfire ammo?

Visit our on-line store for AR Memorabilia

